|
The Behavior of Electrons
Electrons emit photons of light when they are forced out of their 'proper' place in regard to the nucleus and then snap back into place. That 'snap back' releases energy in the form of a photon of light. In response to a number of questions asked about this, here is the following:
As far as electron orbits are concerned, in a fixed ZPE environment, the Bohr atom has orbits at a fixed distance from the nucleus. The orbit radius, a, is a function of the ratio e2/ε where e is the electronic charge and ε is the electrical permittivity of free space and the total orbit energy E. The result is that
The orbit energy E is the sum of the potential energy, T, and kinetic energy, V, of the electron moving around the nucleus. So that
If the math is confusing the issue, you can ignore the section inside the box which follows
The total energy turns out to have exactly the same terms as the kinetic energy, except for a minus sign. Since kinetic energy is ½ mv2, where m is the mass of the electron and v is its velocity in orbit, then it follows that, in a changing ZPE context, any change in electron mass individually, or velocity individually, will change its total orbit energy and hence the orbit radius. If both change simultaneously, the situation is different. This is what happens when the ZPE varies. I put the effect of the ZPE in square brackets to avoid confusion. [Note however, that in the ZPE formulation, throughout the interval between jumps the kinetic energy is conserved, so ½ mv2 is constant. This means that as electron masses, m, increase, the velocity decreases such that v2 is proportional to 1/m. Therefore in the ZPE formulation, the orbit radius stays constant within the interval]. Into this situation, Bohr then injected his “first postulate”. This stated that “Only those orbits occur for which the angular momenta of the planetary electron are integral multiples of h/2π.” Here, of course, h is Planck’s constant. As a result of this stipulation, electron orbit radii go out from the nucleus in a series of jumps. That is to say, they are at fixed distances from the nucleus. Bohr used the quantum number, n, to determine those distances, so that his equation read
The first orbit, the closest one to the nucleus, occurred when n = 1 in equation (6). The next one out had n = 2 in (6). The next orbit out was n = 3, and so on. Thus, Bohr’s quantum condition meant that the angular momentum of an orbit, given by mva (that is mass, times velocity, times radius) went in steps of n times h/2π where n was an integer. The situation looks something like this.
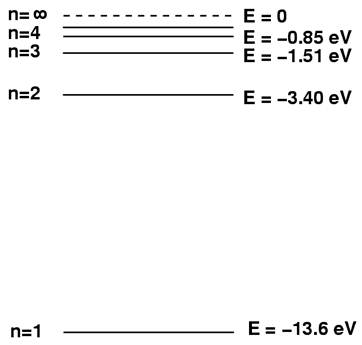
On this diagram, it can be seen that as the quantum number, n, increases the orbits get closer and closer together until at n = infinity ( ∞) we reach the absolute outer limit of the atom’s influence. This effectively marks the outermost possible orbit. On the diagram here, it can be seen that n = 4 and n = ∞ are not that far apart. Note also on this diagram that the energy of an orbit is given in electron volts (eV) and that it is negative. This is what I spoke of in equation (3) and its comment. So for n = 1, it takes 13.6 eV of energy to remove the electron out to n = ∞ where it can escape the atom. This energy is negative because it is energy which must be supplied to get the electron away from the electrostatic attraction of the nucleus. Thus the energy for n = ∞ is zero because no energy needs be supplied to remove the electron from the nucleus. Because of this attraction by the nucleus, electrons tend to “fall” towards the nucleus and emit a photon of energy in the process. The situation is as shown in this diagram.
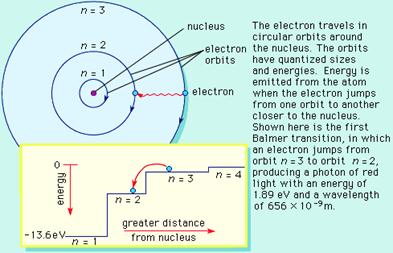
As the electron emits a photon of light falling from an outer orbit to an inner orbit, a series of lines on the spectrum occur. The position of these lines is dependent upon which of the orbits (and the energy) involved. The bluer the light emitted, the higher the energy difference between the orbits involved in the fall of the electron. The situation is like the following diagram:
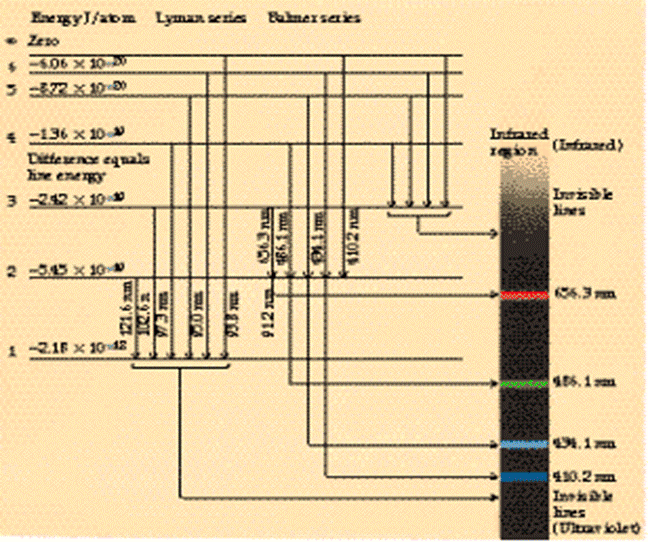
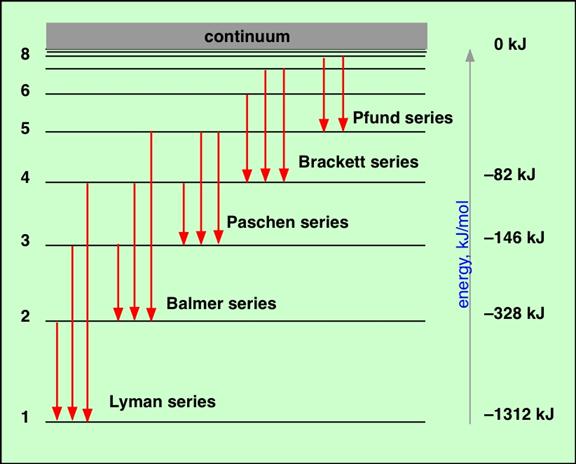
These spectral lines form a series as shown by the above diagram. The diagram below labels the different series specifically. Again the numbers on the left correspond with the quantum number n which represents the orbit quantum numbers. Note that the red arrows show which orbit the electron fell from and the orbit it ended up in.
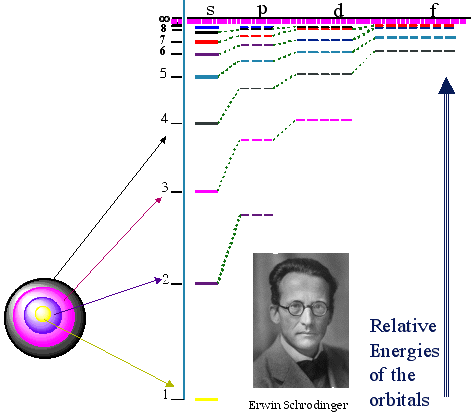
With this background, let us respond to some other questions. “Is there a fixed outer orbit?” Effectively the orbit n = ∞ is the outermost orbit. For any given atom this limit is fixed because it depends on the strength of the electrostatic attraction which is proportional to (e2/ε) for a given atom. The quantity (e2/ε) is absolutely fixed on a changing ZPE scenario. As a consequence, the position of orbit energy E = 0 or, what amounts to the same thing, the orbit n = ∞, remains fixed. Note that for other atoms than hydrogen, there are more than just one proton in the nucleus giving rise to the electrostatic attraction. Therefore, it is usual to express the total electrostatic attraction in the form Z(e2/ε) where Z is the number of protons in the nucleus. Thus for hydrogen, Z = 1. And for any given element, the quantity Z will always be a fixed number, so the attraction is fixed and consequently the position of E = 0 or n = ∞ is fixed. “Are the orbits used from the outermost, and when this one is filled, is then the next inner orbit ‘filled up’?” No. It goes in the reverse. The innermost orbit is filled up first, then the successive orbits moving out. “Can more than one electron use the same orbit at a given time?” Yes, but there is a qualification. Schroedinger made a modification to the Bohr atom. Let us give a diagram here and then explain it.
On this diagram we again have the quantum numbers (n) of the various orbits on the left of the chart. What you will notice is that for n = 1, there is only one orbit. For n = 2 there are in fact two orbits with very slightly different energies. For n = 3, there are in fact three different orbits with very slightly different energies, and so we could go on. These orbits with slightly different energies for the same quantum number, n, are called orbitals. Now here is the key point. Each orbital can only hold two electrons. Thus the first Bohr orbit only has one orbital, so it can only hold a maximum of 2 electrons. The Second Bohr orbit has two orbitals so a maximum total of 4 electrons can exist in the n= 2 orbit. And so we could go on. Each complete Bohr orbit number n is called a “shell.” Thus in the n = 3 shell there are 3 orbitals. Since each orbital hold two electrons, the n = 3 shell holds 6 electrons. There is a reason why two electrons are permitted in each orbital. They must have opposite spin. Yes electrons are spinning and the center of charge is slightly offset from the spin axis. The result is that a magnetic dipole field is created by the spin so each electron acts like a little bar magnet with a north and south magnetic pole. If two electrons have their spin axes arranged in the correct manner, there will be a magnetic attraction between them which will allow them to stay in the same orbital. If their spin axes are such that they repel each other this is not possible. So physicists say that each electron orbital can accommodate two electrons, one with a spin of + ½ , the other with a spin of - ½. “How must I understand electron jumps from one orbit to another? Is this always a jump between adjacent orbits?” No it need not be between adjacent orbits but it can be sometimes. Look again at the diagram above showing the Lyman, Balmer and Paschen series of transitions with the red arrows on the blue-green background. These arrows show which transitions occur to give rise to the various spectral lines. “Does an electron jumping inwards radiate energy (photon) or to the contrary gain energy?” When an electron falls from an outer orbit (higher number of n) to an inner orbit (lower number of n) it radiates the energy difference as an emitted photon of light. This appears as a bright line on the color spectrum. Alternatively, if an electron in an inner orbit is supplied with energy from, say, an X-ray or Gamma-Ray, it will move to an outer orbit or escape. “I understand that an electron is not ‘owned’ by an orbit but that any electron can run in any [designated] orbit.” That is correct. “Can the electron jump around as long as the different orbits are ‘filled’ with the required number of electrons, from the outermost inwards?” An electron will usually stay in its orbital. Unless energy is supplied it will not leave. If there is a supply of electrons and some orbitals are not filled, the ones closest to the nucleus are filled first. Once one set of orbitals is filled making up a quantum number or “shell”, the filling will occur with the next higher quantum number. That is to say the filling proceeds outwards from the nucleus. “You described a general model, but do the figures apply only to the H-atom?” Yes, the general model was described in detail. However, the figures given for orbit energies apply only to the hydrogen atom. So, for example, the 13.6 eV for the n = 1 orbit is correct for hydrogen only. Remember I stated that: “Note that for other atoms than hydrogen, there are more than just one proton in the nucleus giving rise to the electrostatic attraction. Therefore, it is usual to express the total electrostatic attraction in the form Z(e2/ε) where Z is the number of protons in the nucleus. Thus for hydrogen, Z = 1”. Now in Equation (5) on my previous reply there was an equation for the energy of a given orbit. That equation showed that the orbit energy E was given by
In (5) we were talking specifically about the hydrogen atom where Z = 1. For other atoms, the orbit energy is given by
This energy then takes account of the fact that there are additional protons in the nucleus. This means that any given orbit will have an energy that is proportional to the number of protons (Z) in the nucleus. Because of this stronger electrostatic attraction, all orbits will have energy of a greater magnitude, no matter what their number n is equal to. “Are the electron orbits for all elements at the same distance from the nucleus? Consequently, is the n = {infinity} for all elements at the same distance from the nucleus?” For any given element, the quantity Z will always be a fixed number, so the electrostatic attraction, given by Z(e2/ε), is fixed and consequently the position of E = 0 or n = ∞ is fixed for that element. Likewise the position of n = 1 remains the same for that element. However, different elements have different numbers of protons in the nucleus, that is, a different number for Z. Consequently, the electrostatic attraction is different, given by Z(e2/ε), so the orbit energy for any given value of n is also larger in magnitude. It follows, then, that while the n = 1 position is the position of the innermost orbit for any atom, that actual distance from the nucleus will be different from one element to the next. “Are the orbit energies for a certain orbit (say n = 1), the same for all elements, so has orbit n = 1 always the energy of 13.6 eV?” No, because different elements have different numbers of protons and hence different orbit energies as a result as explained above. “Is the Lyman Alpha line specific to the hydrogen atom?” Yes. That line comes from the energy difference between two orbits. There is only one atom with that energy difference between two orbits, namely the hydrogen atom. That energy difference only comes between the n = 2 and n = 1 orbits in the hydrogen atom. In summary we can state that the number of protons in the nucleus determines the orbit energies of a given element and hence the wavelengths of light that element emits as an electron transitions between one orbit and another. Since each element has a number of protons in the nucleus which is unique to that element, the series of spectral lines emitted is also unique to that element. Or in the words of my wife, each element emits light that has its own signature 'bar code.'
|