|
"Common Sense Science" -- electrons and atoms
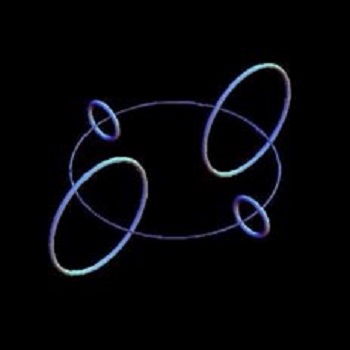
Setterfield: The main basis of the Common Sense Science group is the ring electron. Their model for the atom follows from that premise and the corresponding ring proton. Their claim is that they have followed data to obtain their common sense model of the atom instead of mathematical equations. This is always a desirable aim. But is this claim true? Let us examine a number of key points. Some Reasons for a Different Model Since the 1960’s, there has developed a new branch of physics known as Stochastic Electro-Dynamics (SED physics), This branch of physics explains quantum phenomena and the behavior of subatomic particles as a result of the action of an all-pervasive, universal Zero Point Energy (ZPE). SED physics has been able to explain many quantum phenomena very simply by using classical physics plus the action of the impacting electromagnetic waves of the Zero Point Energy. In the matter under discussion here, Boyer, Puthoff and others have shown that the inward acting pressure of the universal ZPE stabilizes the sphere that carries the electronic charge. So in this case, a ring electron is not necessary. Another basic reason why CSS chose a ring electron instead of a spherical point-like one was that, according to classical physics, an electron orbiting a nucleus would radiate energy. This meant the orbit would degrade, the electron would spiral into the nucleus, and the whole structure would explosively disrupt. This does not happen, of course, and CSS overcomes this problem with an electron which is a stable ring not a point, so no “orbiting” is needed. The ring electron is fixed in position and so does not ‘orbit’ the nucleus. It is usual for quantum physics to attribute the stability of the atom to quantum laws of behavior, but that does not explain why it does not happen. On the other hand, SED physics accepts that classical physics is correct and that a point-like electron is radiating energy as it circles the nucleus. However, a number of SED physicists have shown that, for any given stable electron orbit, the energy radiated by the electron is equal to the energy gained from the action of the ZPE on the electron in that orbit. So, again, in this case, a ring electron is not necessary. The Size and Shape of the CSS Electron and Proton In contrast, actual scattering experiments done in 1984 by Bender show that a vastly smaller radius for the electric charge is mandated, namely about 10-18 meters. This was confirmed in 1992 by a variety of scattering experiments that reveal that the electron indeed had a radius of less than 10-18 meters. But we can go further. The actual shape of the electron was debated by some quantum physicists who proposed that an explanation for the behavior of particles and anti-particles was possible if the electron was not spherical, but had some other shape. On May 26th, 2011, researcher and experimenter Jony Hudson wrote in the journal Nature that about a decade of laser experiments showed the electron was “stunningly spherical.” It was a perfect sphere to 1 part in 1026 centimeters. The statement was made that ”if the electron were magnified to the size of the solar system, it would still appear spherical within the width of a human hair.” The experimental evidence therefore forces the conclusion that the electron is much smaller that the CSS folk predict and it is not toroidal or ring-shaped; rather it really is point-like. The CSS model for the proton is similar to that of the electron, a spinning charged ring, only they propose it is smaller than the electron ring. The CSS folk obtained the size of the electron ring using classical formulae. However, applying the same classical formulation to a proton gives a radius that is significantly bigger than for an electron. Thus an electron should be smaller than a proton if the same formula is used. This also would seem to follow since the electron has 1/1832 times the mass of the proton. If the density of the proton and electron were similar, the electron should once again be smaller. This contrasts with what CSS is proposing, namely that the ring electron is larger than the ring proton. However, if scattering experiments show that the ring model is wrong for the electron, similar scattering experiments suggest it is almost certainly wrong for the proton also. Considering Hydrogen
Yet even here at the simplest level we strike a difficulty. Because one electron and one proton under this arrangement is not a stable entity, CSS claims that an isolated hydrogen atom, H, does not exist. Only the hydrogen molecule, H2, as shown above, or the ionized form, H+, where the electron has been stripped away from the proton, exists. The experimental evidence does not support this contention. It is true that the H-H bond is one of the strongest in chemistry. Therefore it is difficult to get H2 (that is H-H) to dissociate into two hydrogen atoms, that is 2H. However, it does occur. For example, it has been experimentally shown that at 3000 degrees K (4940 F) about 7.85% of hydrogen molecules will dissociate into idividual hydrogen atoms. See for example N.N. Greenwood and A. Earnshaw, Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann (1997). Therefore, the evidence suggests that the CSS contention is incorrect. The CSS Model for the Nuclei of Atoms and Neutrons
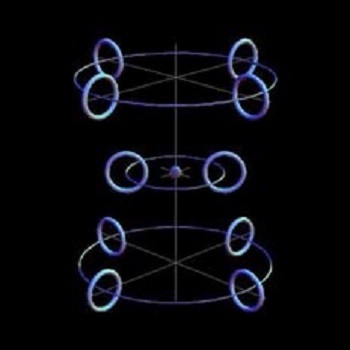
In this diagram, there are a total of 8 electrons and 16 protons in the nucleus. Thus they have 8 protons and 8 neutron-equivalents made up of an electron and proton rather than a neutron as a distinct particle on its own. This illustrates the claim by CSS theorists that neutrons do not exist inside any atomic nucleus, only protons and electrons. The position is strictly held that neutrons are only a proton plus an electron. That is why the hydrogen atom, H, does not exist on their model. Since it, too, is just a proton plus an electron, it would only appear as an unstable neutron apart from any atomic structure. We have shown above that this contention is not supported by experiment and that atomic hydrogen does exist. So, too do neutrons which have different characteristics to atomic hydrogen. There is another point. In the standard model (whose formulae agree with the data), a nucleus contains only protons and neutrons. If neutrons were indeed just a combination of a ring proton and a ring electron, problems would be expected with the nuclei of odd atomic numbers (the sign for 'atomic number' is 'A'). This arises because the particles residing in the nucleus, as experiments have shown, each has its own spin. According to standard modeling, each neutron itself has a specific spin. CSS science agrees that each subatomic particle (they only admit to protons and electrons as being subatomic particles) has its own spin. Within the nucleus of their atoms, there must be a spin which corresponds to the spin of the standard neutron. This would be produced by electron and proton together. But if each had its own spin, there is not any way for a combined spin to be the same as the standard neutron. Why is this spin important? First, if the neutron does not have a spin, it means the behavior of the particles in the nucleus is going to be entirely different from what is observed. In working with atoms, whether it be on a small scale in the lab or with atomic bombs, we have found the nuclei of atoms behave according to certain laws. They require a spin and it is the mathematical calculation of that spin of the neutrons which has made it possible for us to produce such things as atomic energy. Second, if the neutron did not have a spin or if it had a different sort of spin, the nucleus would behave in an entirely different way. This spin is difficult to reproduce with a proton and electron pair as CSS model it. In fact, the extra particles involved in the CSS model for nuclei of odd numbered A would give a different overall spin to the nucleus. This means that nuclei with odd numbered A would obey an entirely different class of spin laws to those actually observed and so be contrary to observation. This is a deep and inherent weakness in the CSS model which is very difficult to overcome. To the best of my knowledge, they have not yet dealt with this. Furthermore, there are two experimentally derived formulae for the binding energy in an atom. "Binding energy" is the energy associated with holding the particles in the nucleus together. In the standard model, the atomic number, A, refers to the total number of protons and neutrons in the nucleus. Experimental data from well over a hundred years indicates that each element has its own atomic number, and thus its own combination of neutrons and protons in its nucleus, as seen in the Periodic Table. Formulation of the binding energy in any atomic nucleus is based on A, its atomic number. However, a straight-forward analysis of the CSS model suggests that the binding energy in an atomic nucleus would have a basic term which was proportional to something other than its atomic number. The CSS people overcome this problem by a clever derivation of another formula which fits the stable isotope data reasonably well for elements with atomic numbers higher than about 30. However, their formula misses the mark for the elements with lower atomic numbers. They do not get the correct predictions for atomic behavior for these common elements. In contrast, the standard formula which acknowledges the existence of neutrons in the nucleus as well as apart from it, predicts the correct results for atoms of high, medium and low atomic numbers. So, on this basis, it seems probable that the CSS modeling of atomic nuclei is incorrect, even though they have made some correct predictions (specifically regarding addional spectral lines for heavier elements). It must be noted, however, that correct predictions do not always indicate a true theory. Physicist Paul Dirac received a Nobel Prize for predicting the existence of the positron, but the theory on which it was based was later proven to be false. Many of Einstein's predictions were correct, but a number of experiments have shown some of the postulates on which they were based were incorrect. Correct predictions are only one measure by which a theory must be proved. By themselves, they do not verify a theory. Other data cannot ignored. The CSS Model for Electron Shells
In Figure 3, the nucleus is shown in the very center of the diagram, with the first shell of two ring electrons in fixed positions around it. The second shell is meant to contain 8 electrons, and this is achieved by stacking two sets of 4 electrons on either side of the first shell. In this way electron shells are built up by the stacking procedure adopted here. It is true that CSS have some physical models built up with magnets which support their view, but this sort of stacked structure is like a human pyramid or a house built of cards, either of which can readily collapse. These stacks of electron rings would collapse with vibration. A rigorous computer modeling of all forces involved would probably reveal this weakness. A Size Difficulty There is a problem with the CSS model related to the size of the nucleus of an atom compared to the size of their proposed electrons. The size of a nucleus can be measured by scattering experiments. What this refers to is firing particles at the nucleus, which are then reflected or bounced off, or deflected by the electrical or magnetic field. This scattering effect tells us the maximum size of the nucleus. The closest approach to a nucleus that was attained by Rutherford scattering was about 5 x 10-15 meters. In addition, all the evidence points to the fact that the nucleus is spherical to within 1%. Look again at the three diagrams above. They were taken from the CSS website and show geometrical arrangements of ring electrons and protons. In the CSS arrangement, the nuclei of the atoms contain a number of ring electrons and somewhat smaller protons. But just one ring electron is claimed by CSS to be 3.86 x 10-13 meters wide! This is almost 100 times the size of the measured nucleus. How can multiple electrons and protons fit into a space 100 times smaller than an electron? As far as I am aware, CSS has not addressed this issue either. As a result of all these considerations, I do not have the confidence that is needed to support the Common Sense Science model, whether for the various atomic nuclei, for the electron shells, or for the electron, proton or neutron themselves. Contrary to their assertion that they have followed the data and used common sense, there are obviously some data they have not noted, and the results are not really intuitive. I am therefore very reluctant to recommend their work in this area. Barry Setterfield, 7th November, 2015.
|