Some Quick Chemistry Basics
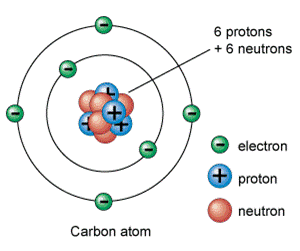
In order to understand some of the things going on in astronomy, it is necessary to understand just a bit of chemistry -- a bit about the elements themselves. And to understand elements, let's take a look at atoms: Every atom has three basic parts. In the middle, called the nucleus, are protons, which have a positive charge, and neutrons, which are neutral. They don't have a charge. Farther out, circling the atom's nucleus, are much, much tinier electrons, which have negative charges. When an atom has as many electons around it as it has protons in the nucleus, then the atom also has a neutral charge.
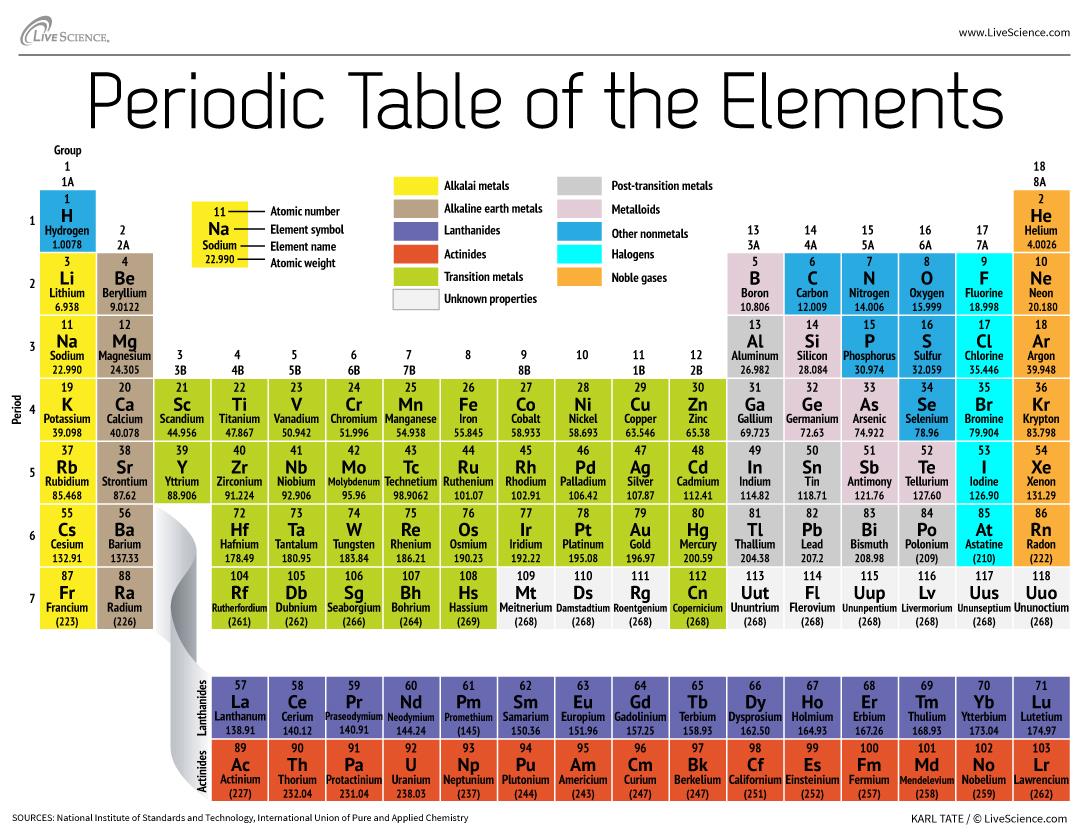
The identity of any atom is determined by the number of protons in its nucleus. The number of electrons or neutrons may vary for different reasons, but it is the number of protons, or positive charges, that define what element that atom is. If there is only one proton in the middle, it is hydrogen. If there are two protons in the middle, it is helium. If there are three protons in the middle, it is lithium. The Periodic Table lines up the elements by how many protons each one has in its nucleus. That number of protons is referred to as that element's "atomic number."
http://media3.s-nbcnews.com/i/newscms/2014_18/416971/140502-science-periodic-table-elements_b2bbb9954b92280ff8011bdcee6e4dcc.jpg Here are what some of the atoms are formed like:
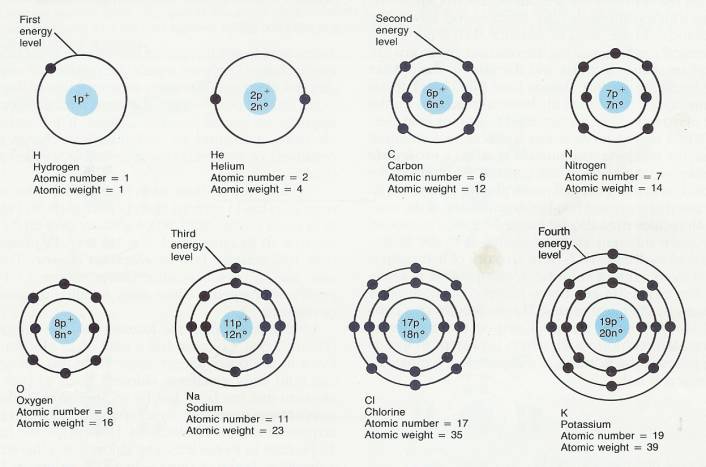
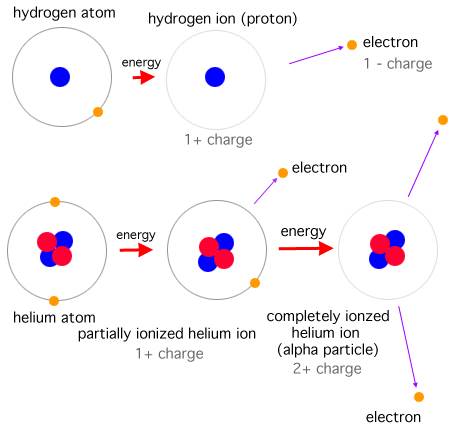
As you can see, the electrons line themselves up in energy levels. The innermost level, level 1, is complete with two electrons. The element with only one electron (and one proton) is hydrogen, the first element on the upper left of the table. Than, all the way to the right, is helium, with two protons and two electrons. A third electron gets shoved into the next 'shell' out. You will see that on the Periodic Table as the first element on the second line, Lithium. The second shell out keeps adding one electron for each proton until it is complete with 8 electrons. You will find that at the far right of the second line. It is Neon. The third shell out is also complete with 8 electrons. After that, it gets more complicated until there are an enormous amount of electrons to match an enormous amount of protons in the nucleus. More about those later. If you look at the different types of atoms above, you can see something interesting. Sodium only has one electron in its third level. But eight would make it stable, and complete. Chlorine has seven in its third level, and eight would also make it complete. So they join, and become sodium chloride. We call it table salt. Hydrogen and oxygen do the same thing to form water. Oxygen has six electrons in its second level and would be complete with eight. Hydrogen has only one electron. So two hydrogen join with one oxygen and there you have H2O, or water. An important thing in astronomy is that one or more electrons can be knocked off an atom when it gets hit by different sorts of energy. Then we have loose electrons wandering around and an atom with more protons than electrons. The atom itself is then called an "ion" and it has a positive charge.
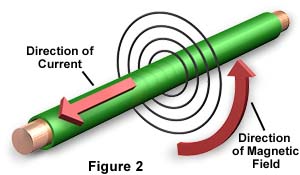
When a stream of ions is traveling in one direction, that is a positive electric current. When a stream of electrons is flowing in one direction, that is a negative electric current. But no matter which kind of electric current it is, it is surrounded by a circling magnetic field. This is why we refer to this as an electromagnetic interaction, or electromagnetism. Electromagnetism is an extremely strong force.
The bigger the atom is, the more electron shells it has, and so the more easily electrons can be knocked off (this is a general rule and has exceptions).
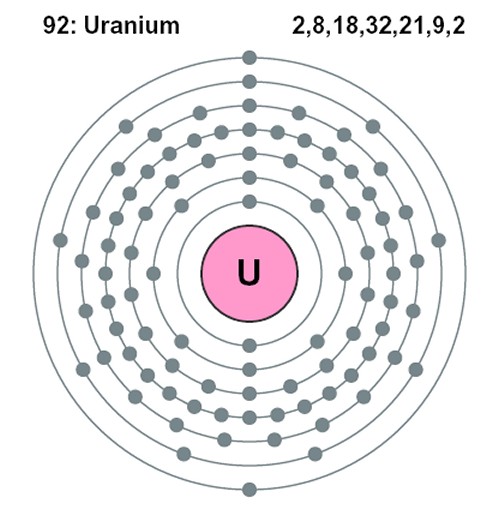
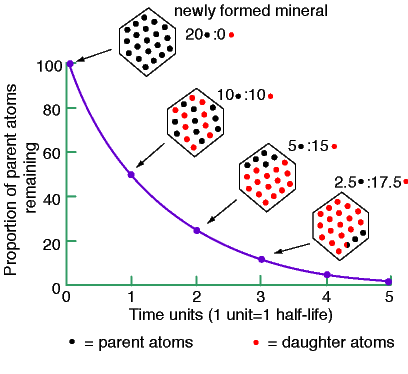
Above is a Uranium atom. It has 92 protons in the middle and about 146 neutrons there as well, trying to keep the protons from pushing each other apart. It has 92 electrons whirling about it, but at much, much farther distances than the diagram shows above. The electrons are WAY out from the nucleus. But you might notice something, as well: although the first and second levels of electrons have the 2 and then the 8 we would expect, as more and more had to pile on to balance the protons in the middle, they shoved a lot more into the third level and the other levels. Uranium, like many of the heavy elements, is very unstable. Not only can it lose electrons, it can,and does, lose pieces of its nucleus through time. Eventually it loses enough to degenerate to a form of lead. This is called radio decay. Thorium will also decay to lead (a different sort of lead); potassium decays to argon, and there are others. The heavy elements which lose bits and pieces (decay) to become other elements are called 'mother' elements, or 'parent' elements.The element each finally turns into is called the 'daughter element.' Radiometric dating is based on how fast a parent element becomes a daughter element. The time is figured on the basis of something called the 'half life' of the decay process 'Half life' refers to the time it takes half of the parent element to become the daughter element. Assuming the rate of decay stays the same, then half of what is left of the parent element will take the same amount of time to turn into the daughter element. Again assuming the rate of decay is constant, half of what is left of the parent element at that point will take just as long to become the daughter element. In this way -- always assumiing the rate of decay has remained constant through time -- by comparing the amount of parent element left in a rock with the amount of daughter element in that rock, the age of the rock can be determined. This is a simple idea of what 'half life' means:
It is generally considered that after five half lives, all -- or just about all -- of the parent element will have become the daughter element. The important point here is that determining the age of something by radiometric dating means one must assume the rate of decay has remained the same through time. However, there are some very strong indications that this assumption is false, and that the rate of decay has changed through time. If the rate of decay was faster in the past, then the rock being dated could be quite a bit younger than supposed. If the rate of decay was slower in the past, then the rock could be a lot older. Some evidence for the change in the rates of decay will be presented in the astronomy course.
|